The world’s first genetically modified spider could lead to new ‘supermaterials’
Researchers funded by the U.S. Navy have used gene-editing technology to make house spiders produce red fluorescent silk. This might seem like a quirky scientific novelty, but the breakthrough is a critical step toward modifying spider silk properties and creating new “supermaterials” for industries ranging from textiles to aerospace. The team at Germany’s University of Bayreuth, led by Professor Thomas Scheibel, successfully applied CRISPR-Cas9—a molecular tool that acts as “genetic scissors” to cut and modify DNA sequences—to spiders for the first time. The study, published in the scientific journal Angewandte Chemie, demonstrates how this technology introduces modifications that enhance the extraordinary properties of spider silk, turning it into a next-generation supermaterial. In a press release, professor Thomas Scheibel, chair of biomaterials at the University of Bayreuth and senior author of the study, said, “Considering the wide range of possible applications, it is surprising that there have been no studies to date using CRISPR-Cas9 in spiders.” His team injected a solution containing CRISPR-Cas9 components into female Parasteatoda tepidariorum, a common house spider species. To facilitate the process, the spiders were anesthetized with carbon dioxide and manually held under a microscope. The solution, which included a gene encoding a red fluorescent protein (called mRFP), was delivered into the eggs within the females’ abdomens before mating with males so the resulting baby spiders could carry the gene modification. [Image: Edgardo Santiago-Rivera and Thomas Scheibel] What are scientists trying to do? The experiment set two objectives: first, to disable a gene called sine oculis, responsible for the development of all spider eyes, in order to study its function. And then second, to insert the fluorescent protein gene into the MaSp2gene, which produces the silk thread spiders use to move hunt, hike, and chill out. In modified specimens, disabling sine oculis caused total or partial eye loss, confirming its critical role in visual development. According to the study, without this gene spiders fail to form eye structures, though the cornea develops normally. But the breakthrough with far-reaching industrial implications is the silk modification. The injected fluorescent protein gene successfully integrated into the MaSp2 gene, causing fibers produced by the modified spiders to glow red under ultraviolet light. [Image: Edgardo Santiago-Rivera and Thomas Scheibel] According to Scheibel, they “have demonstrated, for the first time worldwide, that CRISPR-Cas9 can be used to incorporate a desired sequence into spider silk proteins, thereby enabling the functionalisation of these silk fibres.” He says that the ability to apply CRISPR gene-editing to spider silk is very promising for materials science research—for example, it could be used to further increase the already high tensile strength of spider silk.” This accomplishment was no small feat. Spider genomes are complex, and their embryonic development—marked by unique cell migration stages—complicates genetic editing, according to the researchers. In fact, only 7% of egg sacs that were treated with the CRISPR solution contained modified offspring, a low efficiency rate typical for species with large broods (common house spiders carry about 250 spiders per sac). Additionally, the spiders they used are cannibalistic nature, which required them to be reared in isolation (not all spiders are cannibalistic in nature, but many do eat their males after mating and others eat each other). [Image: Edgardo Santiago-Rivera and Thomas Scheibel] The race for “super silk” It’s a very promising development indeed. Spider silk is one of nature’s strongest materials. Certain types of spider silk are significantly lighter and tougher than Kevlar. Silk is also far more elastic, which means it can stretch and return to its original shape without losing its strength. To top all this, spider silk production by spiders (or other animals, more on this later) does not involve the industrial processes, high energy consumption, and pollution associated with the manufacturing of synthetic materials like Kevlar. This is a major area of interest for biomimicry and sustainable materials. Until now, modifying spider silk’s properties required costly, lab-based post-extraction processing, which is difficult to scale. This study shows that altering silk directly within the organism is feasible, paving the way for custom-designed silks with enhanced properties. While spider silk remains unmatched in natural performance, CRISPR-edited silkworms are emerging as scalable alternatives. Silkworms can be farmed en masse (unlike solitary, cannibalistic spiders), and recent advances show their engineered silk reaches 1.3 GPa tensile strength, comparable to high-tensile steel, which is a steel alloyed with chromium, molybdenum, manganese, nickel, silic

Researchers funded by the U.S. Navy have used gene-editing technology to make house spiders produce red fluorescent silk. This might seem like a quirky scientific novelty, but the breakthrough is a critical step toward modifying spider silk properties and creating new “supermaterials” for industries ranging from textiles to aerospace.
The team at Germany’s University of Bayreuth, led by Professor Thomas Scheibel, successfully applied CRISPR-Cas9—a molecular tool that acts as “genetic scissors” to cut and modify DNA sequences—to spiders for the first time. The study, published in the scientific journal Angewandte Chemie, demonstrates how this technology introduces modifications that enhance the extraordinary properties of spider silk, turning it into a next-generation supermaterial. In a press release, professor Thomas Scheibel, chair of biomaterials at the University of Bayreuth and senior author of the study, said, “Considering the wide range of possible applications, it is surprising that there have been no studies to date using CRISPR-Cas9 in spiders.”
His team injected a solution containing CRISPR-Cas9 components into female Parasteatoda tepidariorum, a common house spider species. To facilitate the process, the spiders were anesthetized with carbon dioxide and manually held under a microscope. The solution, which included a gene encoding a red fluorescent protein (called mRFP), was delivered into the eggs within the females’ abdomens before mating with males so the resulting baby spiders could carry the gene modification.

What are scientists trying to do?
The experiment set two objectives: first, to disable a gene called sine oculis, responsible for the development of all spider eyes, in order to study its function. And then second, to insert the fluorescent protein gene into the MaSp2gene, which produces the silk thread spiders use to move hunt, hike, and chill out. In modified specimens, disabling sine oculis caused total or partial eye loss, confirming its critical role in visual development. According to the study, without this gene spiders fail to form eye structures, though the cornea develops normally.
But the breakthrough with far-reaching industrial implications is the silk modification. The injected fluorescent protein gene successfully integrated into the MaSp2 gene, causing fibers produced by the modified spiders to glow red under ultraviolet light.
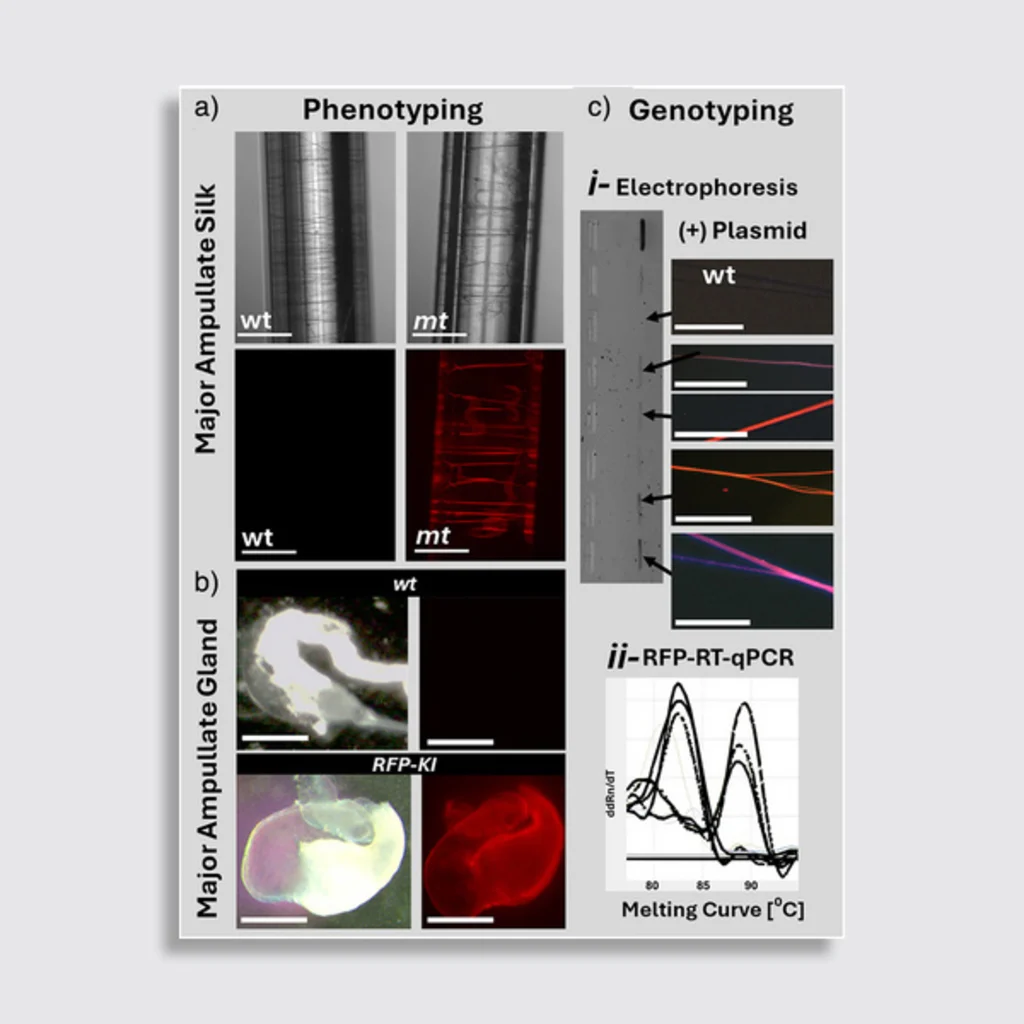
According to Scheibel, they “have demonstrated, for the first time worldwide, that CRISPR-Cas9 can be used to incorporate a desired sequence into spider silk proteins, thereby enabling the functionalisation of these silk fibres.” He says that the ability to apply CRISPR gene-editing to spider silk is very promising for materials science research—for example, it could be used to further increase the already high tensile strength of spider silk.”
This accomplishment was no small feat. Spider genomes are complex, and their embryonic development—marked by unique cell migration stages—complicates genetic editing, according to the researchers. In fact, only 7% of egg sacs that were treated with the CRISPR solution contained modified offspring, a low efficiency rate typical for species with large broods (common house spiders carry about 250 spiders per sac). Additionally, the spiders they used are cannibalistic nature, which required them to be reared in isolation (not all spiders are cannibalistic in nature, but many do eat their males after mating and others eat each other).

The race for “super silk”
It’s a very promising development indeed. Spider silk is one of nature’s strongest materials. Certain types of spider silk are significantly lighter and tougher than Kevlar. Silk is also far more elastic, which means it can stretch and return to its original shape without losing its strength. To top all this, spider silk production by spiders (or other animals, more on this later) does not involve the industrial processes, high energy consumption, and pollution associated with the manufacturing of synthetic materials like Kevlar. This is a major area of interest for biomimicry and sustainable materials.
Until now, modifying spider silk’s properties required costly, lab-based post-extraction processing, which is difficult to scale. This study shows that altering silk directly within the organism is feasible, paving the way for custom-designed silks with enhanced properties.
While spider silk remains unmatched in natural performance, CRISPR-edited silkworms are emerging as scalable alternatives. Silkworms can be farmed en masse (unlike solitary, cannibalistic spiders), and recent advances show their engineered silk reaches 1.3 GPa tensile strength, comparable to high-tensile steel, which is a steel alloyed with chromium, molybdenum, manganese, nickel, silicon, and vanadium. Companies like Kraig Biocraft Laboratories already use CRISPR to produce spider-silk hybrids in silkworms, targeting industries like textiles and medical sutures.
However, spider silk holds unique advantages over those genetically modified silkworms. Its dragline fibers are inherently stronger and 10 times finer. Using the method developed by Scheibel’s team, potential CRISPR-enhanced spiders are likely to gain more superpowers, like getting closer to Kevlar or gaining better electrical conductivity.
Where super silk might be used
In medicine, spider silk’s biocompatibility makes it ideal for dissolvable surgical sutures that reduce scarring and artificial tendons mimicking natural elasticity. Researchers are also developing 3D-printed scaffolds infused with silk proteins to regenerate bone or cartilage, leveraging silk’s porous structure to support cell growth. For drug delivery, silk microcapsules could release medications at controlled rates, improving treatments for chronic diseases. New applications can integrate silk in sensors for real-time health monitoring in implants or conduct electricity for flexible electronics.
The U.S. Navy’s funding of the research makes sense too, given its interest in lightweight body armor. Spider silk can outperform Kevlar, while its elasticity reduces blunt-force trauma. In aerospace, silk composites could replace carbon fiber, cutting aircraft weight by 40% and improving fuel efficiency. NASA already explores silk-based materials for radiation shielding in space habitats, capitalizing on its strength-to-weight ratio.
Companies like AMSilk and Spintex engineer spider silk proteins into biodegradable textiles, reducing reliance on synthetic fabrics derived from fossil fuels. Adidas has prototyped ultralight running shoes with silk midsoles, while Airbus tests silk-based cabin panels to lower aircraft emissions. Spintex claims that its energy-efficient spinning process—1,000 times more efficient than plastic production—could revolutionize sustainable fashion, addressing the industry’s 10% global carbon footprint.
Right now, Scheibel’s team is already exploring CRISPR edits to add moisture-responsive shrinking or toxin-detecting color changes to silk.
Once they achieve whatever new wundersilks they—or the U.S. Navy—have in mind, they will have to come up with a way to mass-produce them. This evokes images of farms full of millions of genetically modified spiders, which sounds as fun as a rave with 10,000 zombies from The Last of Us. But the spider farms may never happen: As the researchers mention, many spiders are cannibals and the success rate of modification is still very low, so this will be a challenge. That is what makes genetically modified silkworms ideal to make spider-like silks, as they have been farmed for silk production since the neolithic, about 6,000 years ago, when Yangshao culture in China realized that silkworms could be raised to harvest cocoons that then got weaved to create silk fabric.
The solution may be taking the successful spider DNA modifications they develop and using other animals to produce them, like silkworms or goats (yes, spider-goats are a thing). I’ll leave you at this point. Good luck in your dreams tonight, my arachnophobic friends.





































































































![Building A Digital PR Strategy: 10 Essential Steps for Beginners [With Examples]](https://buzzsumo.com/wp-content/uploads/2023/09/Building-A-Digital-PR-Strategy-10-Essential-Steps-for-Beginners-With-Examples-bblog-masthead.jpg)




















































